A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor
Exposé zum Thema: eCTD - Neue Wege der elektronischen Arzneimittelzulassung und die Vernetzung elektronischer regulatorischer P

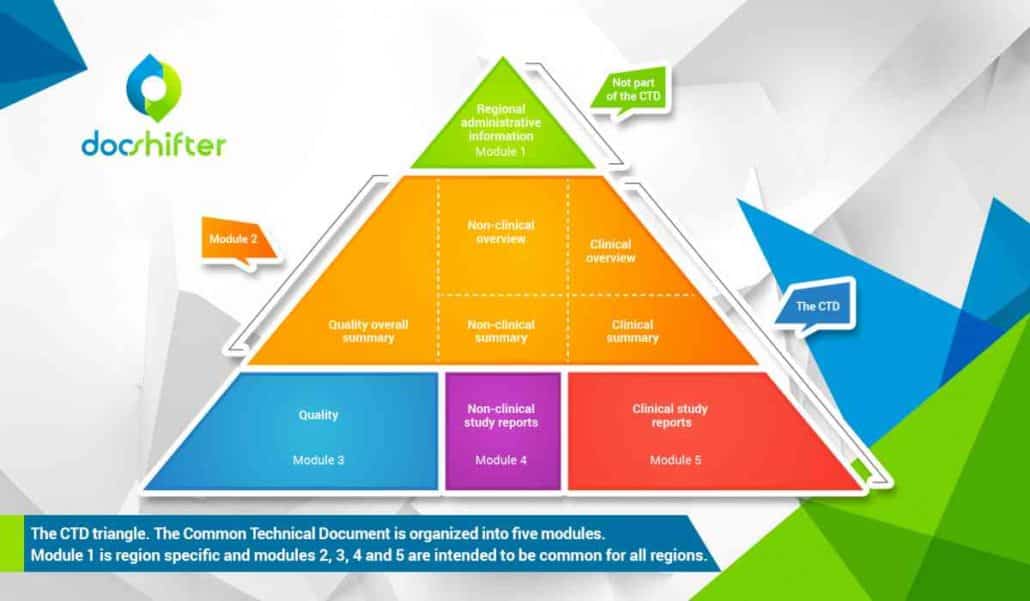
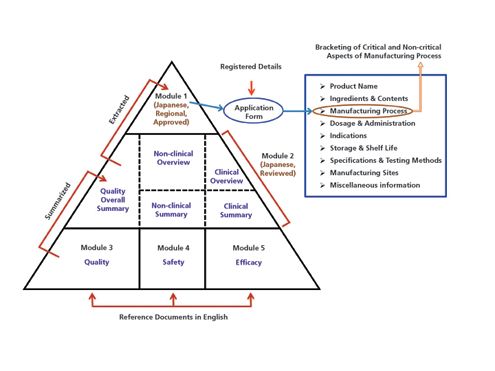
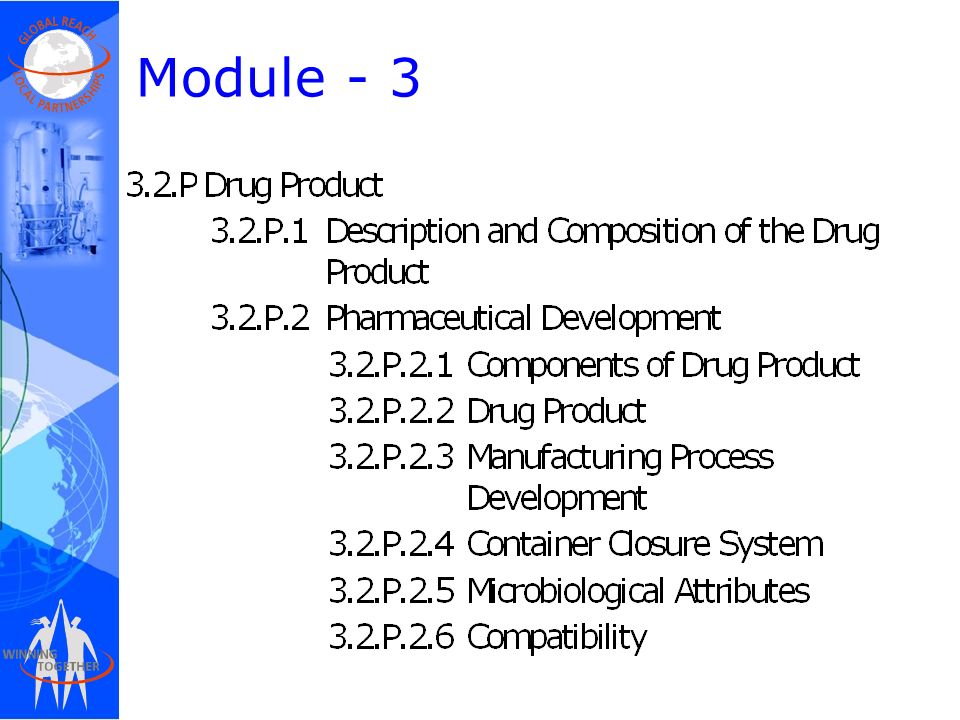
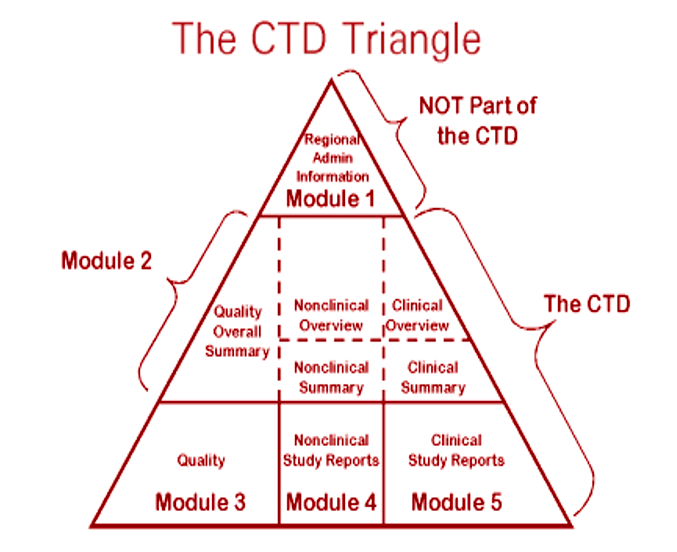
ClinProve - CTD Structure The Common Technical Document is divided into five modules: 1. Administrative and prescribing information 2. Overview and summary of modules 3 to 5 3. Quality (pharmaceutical documentation) 4.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format | Zenosis – Learning for Life
WHO Guidelines on submission of documentation for the pilot procedure for prequalification of similar biotherapeutic products fo