Guideline on good pharmacovigilance practices (GVP) Module VI – Management and reporting of adverse reactions to medicinal pro

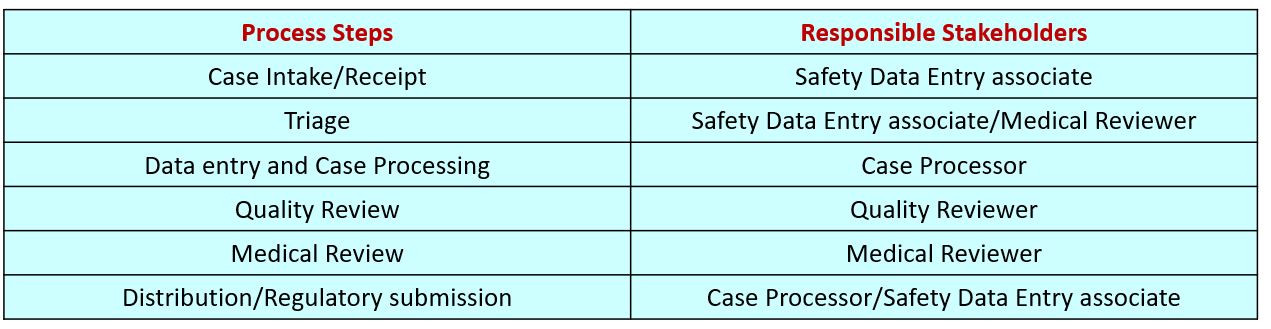
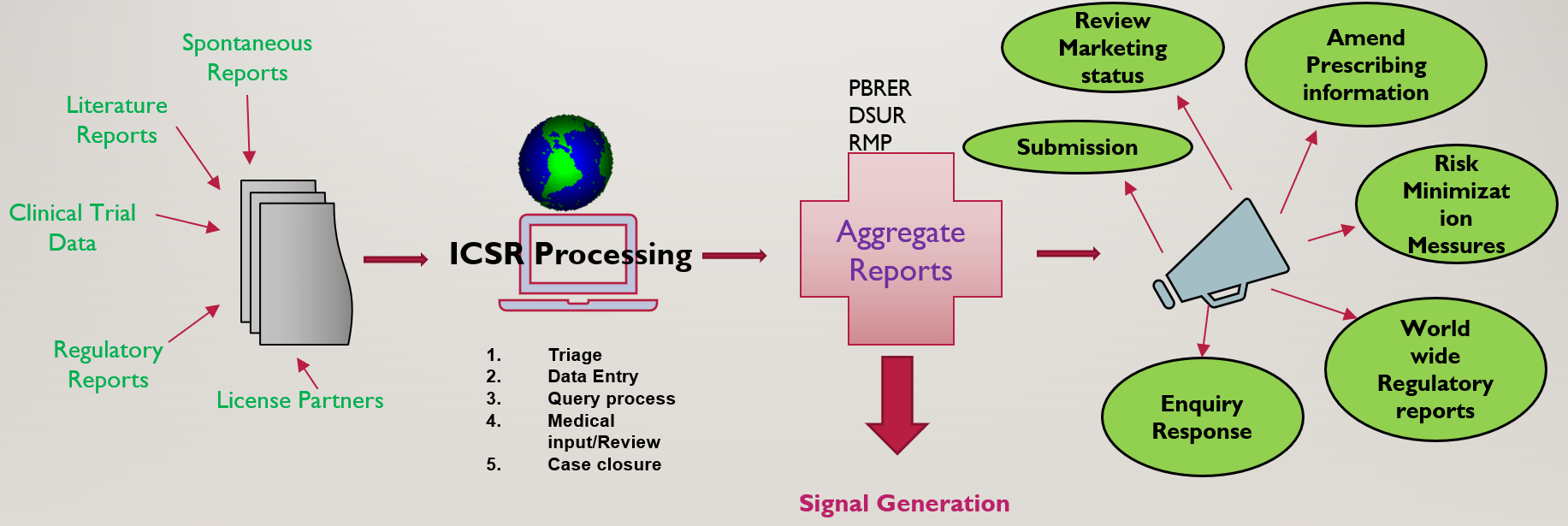
Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance - Marina A. Malikova, 2020

Practical applications of regulatory requirements for signal detection and communications in pharmacovigilance - Marina A. Malikova, 2020

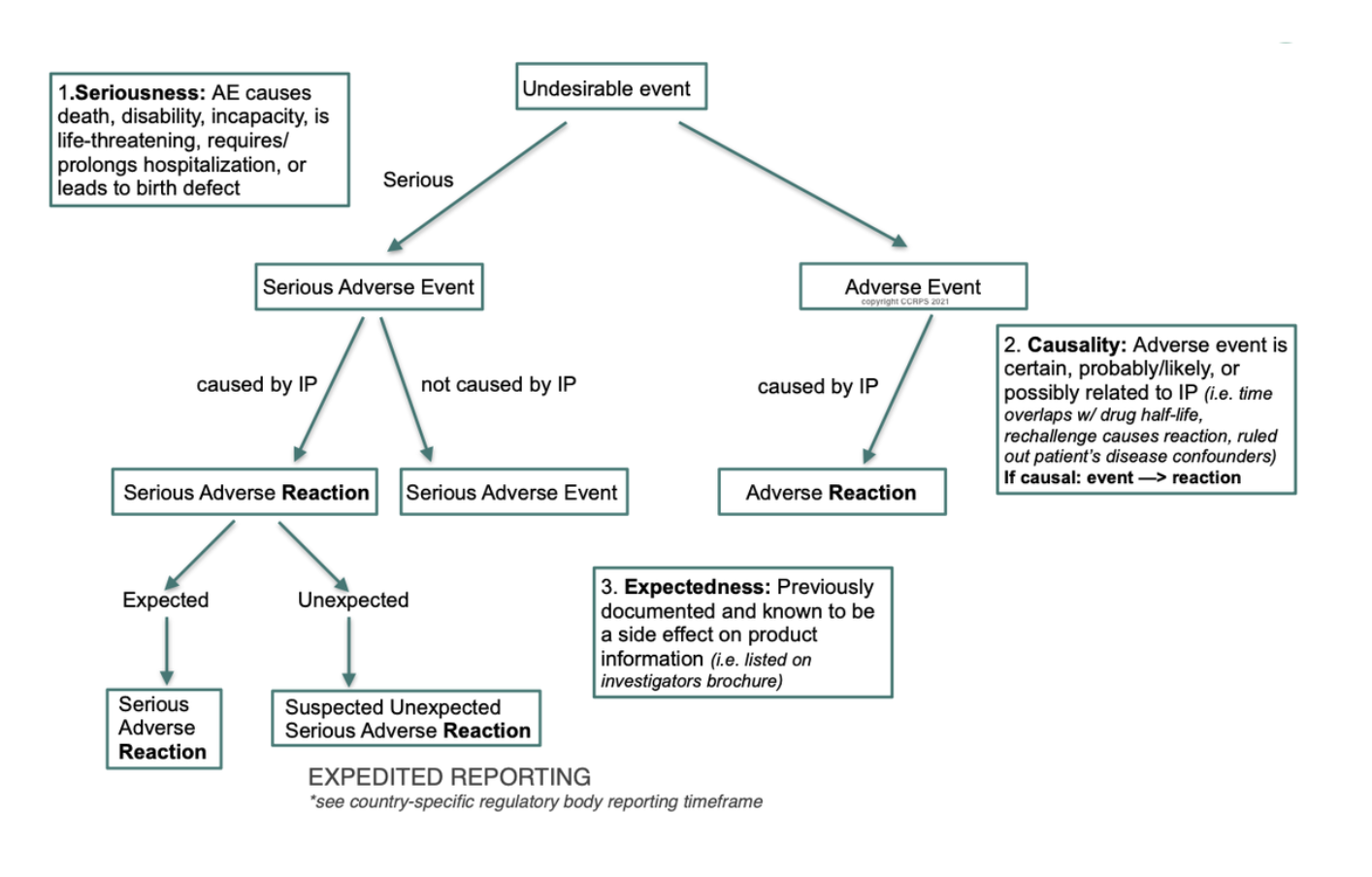
solicited and unsolicited reports in pharmacovigilance — Clinical Research Certification I Blog - CCRPS

Reporting adverse reactions to marketed health products - Guidance document for industry - Canada.ca

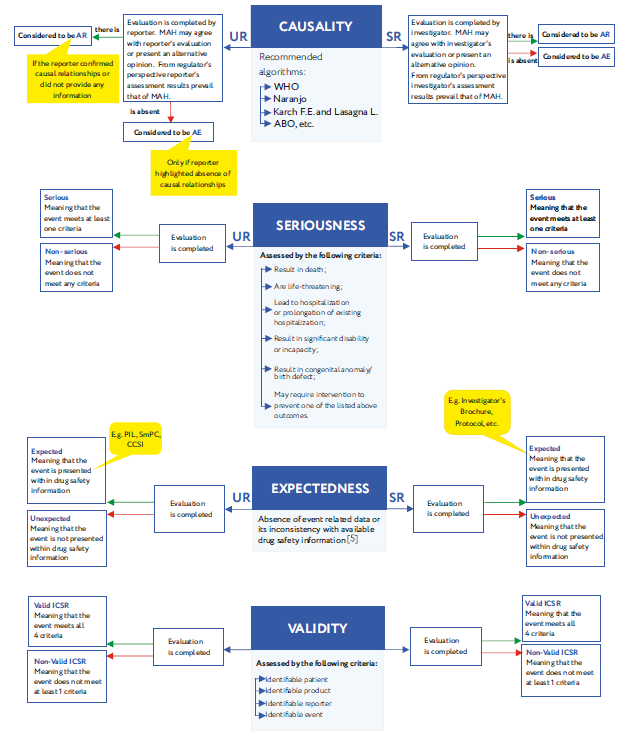
Pharmacovigilance Case Handling Training and Courses - GxP Training : Certified Online Courses for Life Sciences